Life Science and Medicine
Artificial Intelligence plays a transformative role in Medical Informatics, Life Science and Medicine, particularly in handling and analyzing large-scale data sets. AI technologies like machine learning algorithms can analyze vast amounts of medical data quickly, including Electronic Health Records (EHR), genetic information, bio-signals, and patient-generated data. Here, AI can assist in identifying patterns and insights that might be missed by human analysis, leading to improved diagnostic tools, personalized medicine, and better patient outcomes. We strive to bring current AI models closer to the clinics and provide the infrastructure of tomorrow to set the interoperability standards that will enable AI analyzes on large-scale clinical data.
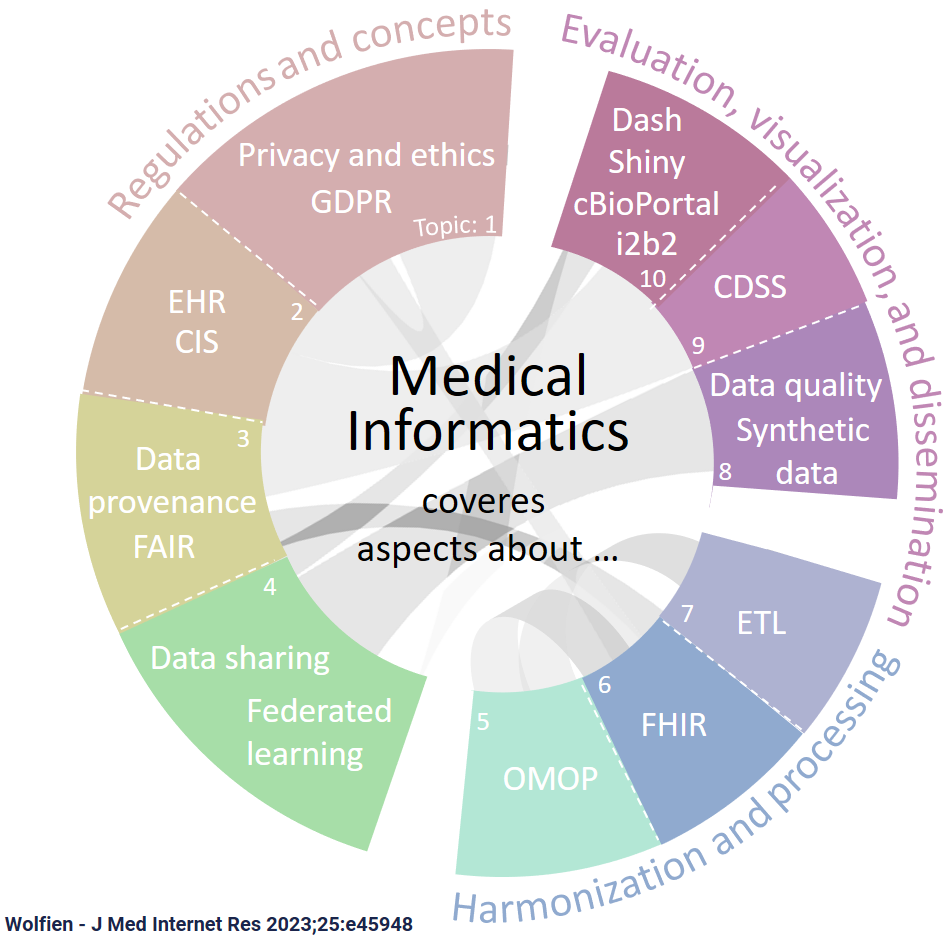
Research Focus
Synthetic tabular data for artificial clinical trials
Synthetic tabular data for artificial clinical trials involves creating realistic but artificial datasets that closely mimic real-world clinical trial data. This approach uses AI algorithms, especially generative models (e.g., CTAB-GAN, NFlow) or transformer models, to generate data resembling actual patient information in structure and statistical properties. This synthetic data helps overcome privacy concerns and data availability issues or improves the investigation of missing control arms and alternative study cohorts among other clinical applications. It also allows researchers to independently test hypotheses, validate models, and simulate outcomes without relying on actual patient data, thus accelerating research while maintaining patient confidentiality.
Biomarker predictions based on genomic sequencing data
Biomarker prediction using AI algorithms leverages the complex patterns in genomic sequencing data. These advanced AI models, based for example on GAN or transformer architectures, can identify potential biomarkers by learning intricate genomic sequences and their associations with various diseases or therapeutic responses. We are utilizing these concepts on our current clinical application of lung cancer patients and mainly seek to investigate long-range dependencies in genetic sequences, as well as large DNA translocations. These approaches may open up new avenues in precision medicine, offering insights into cancer mechanisms in general, as well as individual drug development strategies for lung cancer.
Biosignal processing for sleep research
We are developing AI models for automated sleep scoring from biosignals, such as electroencephalograms and electrocardiograms. Our aim to support tedious manual scoring tasks in clinical routines by training AI models to support these tasks. We are also investigating the limitations of AI scoring algorithms to understand where AI is safe to use. We are further using AI to answer neurological research questions about different sleep states. Additionally, we are exploring where AI can reduce the complexity of measurement setups by improving the analysis of information from simplified setups, such as wearables. We will also transfer this AI knowledge and methods to the assessment of Anaesthesia from biosignals.
Standardization of clinical data for AI applications
Our standardization efforts of clinical data for AI applications involve harmonizing diverse datasets into a common format, enhancing their utility in AI-driven healthcare projects. The Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) and tools from the Observational Health Data Sciences and Informatics (OHDSI) initiative, like the Patient-Level Prediction (PLP) and the Health Analytics Data-to-Evidence Suite (HADES), play a key role in this process. These tools and models facilitate the conversion of varied clinical datasets into a standardized structure, enabling efficient data analysis, interoperability across different healthcare systems, and more robust AI model development. This standardization is an essential foundation for leveraging the full potential of AI in healthcare analytics and research.
Aims
In the research area “Life Science and Medicine”, our strategic goals encompass leveraging AI for enhanced data analysis to ultimately improve clinical decision-making. This is coupled with the use of AI in various fields, ranging from genetic biomarker prediction to foster personalized medicine and enable early disease detection. A foundational aspect of these goals is the standardization and harmonization of clinical data, utilizing standard formats like to ensure compatibility and reliability in AI applications. Expanding these AI methodologies to broader medical fields, such as Anaesthesia assessment, lung cancer or Acute Myeloid Leukaemia further exemplifies the commitment to integrating advanced AI tools in medical research and practice.






